
Parental Note: This experiment is geared towards ages 8 and up.
Additional Note: This experiment will take roughly one hour to set up and three days to complete. Assistance may be required to boil an egg.
Experiment Overview:
You may have already learned from the air quality experiment that pollution from burning fossil fuels causes smog and can cause people to have asthma and other breathing problems. Another problem associated with air pollution is that it creates acid rain.
Pure distilled water is a neutral solution with a pH of 7. An acid is a chemical substance that has a high level of hydrogen ions and a pH lower than 7. When pollution is released into the atmosphere from burning fossil fuels, nitrogen oxide and sulfur dioxide contained in the polluted air react with the water in the atmosphere to create nitric and sulfuric acid. This combination creates acid rain, which is any type of acidic precipitation (rain, snow, hail, etc.) that falls back down to Earth.
A small amount of acid rain is due to natural causes like volcanoes and wildfires, but most of the acid rain we experience here on Earth is due to human causes such as burning fossil fuels in factories and motor vehicles. Acid rain can have harmful effects on plants, animals, and humans, and can even damage man-made structures. Acid dissolves calcium, which is an important element that can be found in the rocks that we make buildings and statues with (such as limestone). Calcium is also found in the shells and skeletons of many living things (such as insects, crabs, and snails). When acid rain falls and is absorbed by the soil, it can remove calcium and other nutrients from the ground and stunt the growth of trees. Acids can also erode (wear away) metals such as copper and aluminum.
The damage caused by acid rain often takes several years to develop.
In this experiment, you will collect a few living and nonliving items from around your home and test how each one reacts to being immersed in distilled water for three days against being immersed in an acid for five days.
Before beginning, think about the following questions and write down your predictions:
- What do you think will happen to the paper clip in the water? How about in vinegar?
- What do you think will happen to the leaf in water? How about in vinegar?
- What do you think will happen to the eggshell in water? How about in vinegar?
Experiment Materials:

- Two glass jars
- Two paper clips
- Two equal-sized leaves from the same tree
- One hard-boiled egg
- Distilled white vinegar
- Distilled water
- Tape
- One Sharpie marker
- Notepad
Experiment Process:
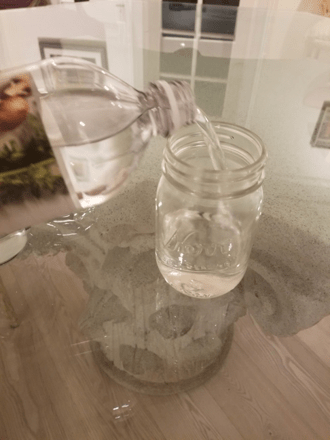
Step 1
Fill one jar ¾ full with distilled water and label it “distilled water” with a piece of tape and a sharpie marker. Fill the other jar ¾ full with vinegar and label it “vinegar” with a piece of tape and a sharpie marker.

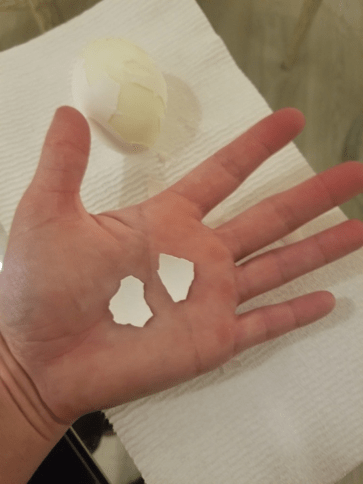
Step 2
Peel the egg shell from your hard-boiled egg and break off two equal-sized egg shell pieces.

Step 3
Label each card with a number and location. You may want to write “Science experiment in progress; please do not touch” on the index cards so no one removes them.


Step 4
After one day, compare the appearance of the items in the water to the appearance of the items in the vinegar. Write down your observations.

Step 5
Repeat step four each day for a total of five days. On the last day, write down your conclusions.
Share Your Results
- Did you observe any changes to the leaf, paper clip, and eggshell that you immersed in the distilled water?
- Did you observe any changes to the leaf, paper clip, and eggshell that you immersed in vinegar?
Conclusions:
Burning fossil fuels in factories and vehicles cause water in the atmosphere to become more acidic. When that water falls as precipitation, the acidity can cause damage to both living and nonliving things on Earth. As you may have observed, in just a few days strong acids have the ability to dissolve the protective shell of an egg, cause aluminum to rust and can cause damage to the pigment cells in leaves, making it hard for them to photosynthesize.
Extension:
In real life, these changes do not happen as rapidly as you observed by fully immersing objects in acid. These changes happen slowly over time through repeated exposure to droplets of acid rain. You can try a variation of this experiment that involves using one spray bottle filled with distilled water, and another filled with acid. Rather than immersing the objects in the liquids in a jar, you can spray them with the liquids for 1 minute each day. Continue this experiment each day until you start noticing any changes in the appearance of the objects.









